Forms products with higher bond energy than reactants c. CH4N2H2--CH3NNH3 Which statement about the reaction is correct.
What Are Some Safe Exothermic Reactions That Occur With Or In Presence Of Water Quora
Releases heat and light.
. Decomposition reactions are exothermic in nature so release heat. We can not tell anything about kinetic energy. Which phrase best describes.
δh for an endothermic process is _____ while δh for an exothermic process is _____. Releases more energy than it absorbs. Let me first reveal the identity of your salts.
The rearrangement of atoms by breaking and reforming chemical bonds a reaction that absorbs heat from the surroundings a reaction that releases heat to the surroundings the rearrangement. Determine PH2t for this reaction at the same time. The energy of the reactants is lower than the energy of the product and the reaction is endothermic.
Releases more energy than it absorbs. Does not absorb any energy b. An example of an exothermic reaction is the chemical reaction between sodium and chlorine which results in the formation of sodium chloride also known as common salt.
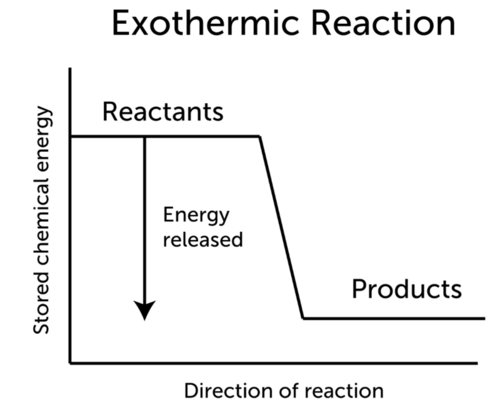
The graph below represents a chemical reaction. An exothermic reaction refers to a physical or chemical reaction that releases heat. The chemical energy potential energy of reactant is higher than energy of product in exothermic reaction while chemical energy potential energy of reactant is less than energy of product in endothermic reaction.
The reverse reaction is exothermic. AEach reactant has more chemical potential energy than each product BIt takes more energy to form C-H bonds in the reactants than is released from C-H bonds in the products CMore chemical potential energy is stored in the bonds of CH4 than in the bonds of. This reaction is best described as.
Hope it will be helpful. Which best explains why nuclear reactions release more energy than chemical reactions. An exothermic reaction occurs when energy is released during a chemical reaction often times when chemical bonds are made.
What describes an exothermic reaction. Endothermic Reactions The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. Obvious if the activation energy is known.
A chemical reaction that releases more energy than it absorbs. Which best describes a chemical reaction. Salt A is ammonium nitrate and Salt B is calcium chloride.
In endothermic reaction heat. Endothermic because energy is released. Which of the following statements describes the difference between endothermic and exothermic chemical reactions.
Exothermic because energy is. One of your salts generated an endothermic reaction with water while the other salt generated an exothermic reaction with water. For the overall chemical reaction shown below which one of the following.
The chemical reaction shown is exothermic. Of reactant molecules are increased the rate of reaction increases. Is in a state of equilibrium d.
Thats because you were given two different salts. If the energy of the reactant is higher than the energy of the product the reaction is exothermic. Explaination- Endo means within and thermic relates to energy.
View the full answer. What is the term for the amount of energy that needs to be added for a chemical reaction to start. If an exothermic reaction is reversed it becomes an endothermic reaction.
For the given reaction. So potential energy of product is less than potential energy of reactant. Which phrase best describes an exothermic chemical reaction.
Endothermic because energy is absorbed. Which phrase best describes an exothermic chemical reaction. 2H2O22H2O The chemical energy of hydrogen and oxygen is greater than the chemical energy of water.
Which of the following is true about endothermic reactions. Exothermic because energy is absorbed. Which statement best describes the difference between xylem and phloem.
In exothermic reaction heat is released from the system to the surroundings thereby temperature of the surroundings increases. 1 - Energy is absorbed in endothermic reactions but is released in exothermic reactions. So option a is correct.

For An Exothermic Chemical Process Ocuuring In Two Process Occuring In Two Steps As Youtube
Exothermic Reaction Ck 12 Foundation

Exothermic And Endothermic Processes Introduction To Chemistry
0 Comments